
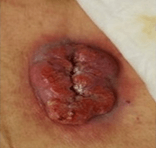
Case Study: Fungating Basal Cell Carcinoma
Patient History

- 75-year-old male with basal cell carcinoma
- Diabetes
- Coronary artery disease
Treatment

- Wide location excision of basal cell carcinoma
- Application of MirraSurgTM and secondary dressing
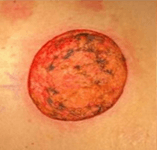
- Secondary dressing change, residual MirraSurg
- moistened with saline with secondary dressing
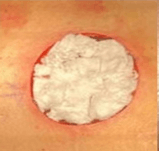
- Fully granulated at 3 weeks
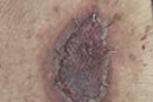
- Definitive closure with autologous skin graft
application