
Clinical Evidence
The latest research on Mirragen® and how effective it can be on a variety of wounds.
2025 Multicenter Randomized Controlled Trial
In a first-of-its-kind study amongst synthetic skin substitutes, Mirragen, a borate-based bioactive glass Fibre Matrix (BBGFM), demonstrated significantly improved wound healing at 12 weeks in Wagner Grade 1 DFUs compared to SOC, consistent with a previous pilot study and should be considered in the treatment arsenal for these types of chronic ulcers.
Key Results
Primary Endpoint (Per Protocol)
Patients achieving complete wound closure:

Mirragen + Standard of Care
N=44 Patients

Standard of Care
N=38 Patients
Primary Endpoint (Modified Intent-to-Treat)
Patients achieving complete wound closure:

Mirragen + Standard of Care
N=67 Patients

Standard of Care
N=66 Patients
2021 Multicenter Randomized Controlled Trial
Study comparing Mirragen plus SOC (Standard of Care) to SOC alone for the treatment of diabetic foot ulcers. After two weeks of screening, patients are randomized for 12-week treatment. For this patient population, Mirragen had the greatest impact during first 4 weeks of treatment.1
79.4% vs. 36.5%

Mirragen doubled the SOC in wound area reduction (79.4% vs 36.5%)
2.8x

Mirragen increased wound closure 2.8x vs. SOC
0 Infections

SOC had 5 patients withdrawn due to infection, Mirragen had 0 adverse events related to infection of the index ulcer.
In an RCT, investigators concluded that angiogenesis in the Mirragen-treated group was “probably rapid because wound area reduction on average is high over the first week”.1
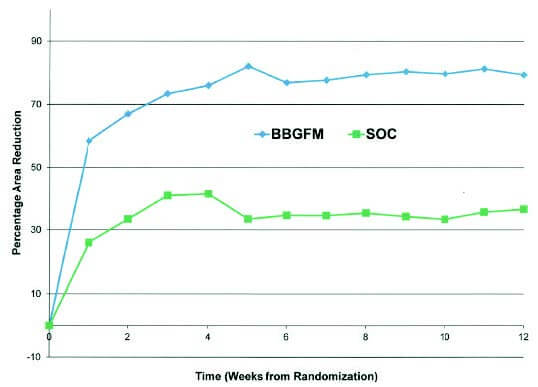
Weekly percentage wound area reduction by treatment group (From Armstrong et al. 2021, Figure 4).
Versatile Form Factor



1 Armstrong DG, Orgill DP, Galiano RD, et al. A multi-centre, single-blinded randomized controlled clinical trial evaluating the effect of resorbable glass fibre matrix in the treatment of diabetic foot ulcers. Int Wound J. 2021;1-11.